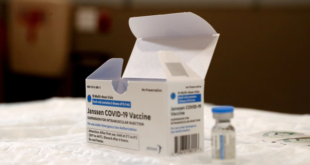
The European Union’s medicines regulator on Tuesday said it had found a “possible link” between Johnson & Johnson’s (J&J) COVID-19 vaccine and the development of rare blood clots in a small number of recipients, but concluded the shot’s overall benefits outweigh any risks associated with its use.
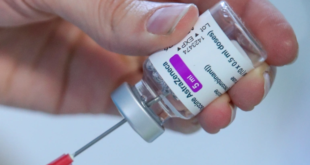
Read More | Lire La SuiteGermany, France, Italy and Spain halt use of AstraZeneca vaccine
Suspensions follow reports that some people developed blood clots after receiving the shot, but the WHO and the EMA say they have seen no evidence of a link.
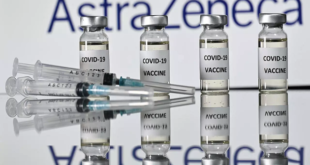
Read More | Lire La SuiteLe régulateur européen approuve le vaccin AstraZeneca pour les plus de 18 ans
L'Agence européenne des médicaments (EMA) a recommandé, vendredi, d'accorder une autorisation de mise sur le marché du vaccin d'AstraZeneca contre le Covid-19 pour les personnes âgées de 18 ans et plus. Il s'agit du troisième vaccin contre le Covid-19 dont l'EMA a recommandé l'autorisation.
Read More | Lire La Suite



 World Opinions Débats De Société, Questions, Opinions et Tribunes.. La Voix Des Sans-Voix | Alternative Média
World Opinions Débats De Société, Questions, Opinions et Tribunes.. La Voix Des Sans-Voix | Alternative Média